The total number of trials disrupted by COVID-19 since June has been falling slowly, while trials impacted by slow enrollment continue to increase. For trials currently affected by slow enrollment, one-tenth of these are specifically due to the unavailability of sites and investigators, says GlobalData, a leading data and analytics company.
Brooke Wilson, Trials Intelligence Associate Director at GlobalData, comments: “Many hospitals that serve as trial sites were inundated with COVID-19 patients at the start of the pandemic and may still not be available; likewise, many investigators may have been reassigned to COVID-19 drug discovery trials or treating COVID-19 patients, or the activation of sites for non-COVID-19 trials is being deprioritized.”
Another concern for slow enrollment is due to subjects being high-risk. Therefore, they may be unwilling to enroll in a clinical trial if they have a serious chronic or acute condition that affects their immune system, giving them a greater chance of contracting severe COVID-19.
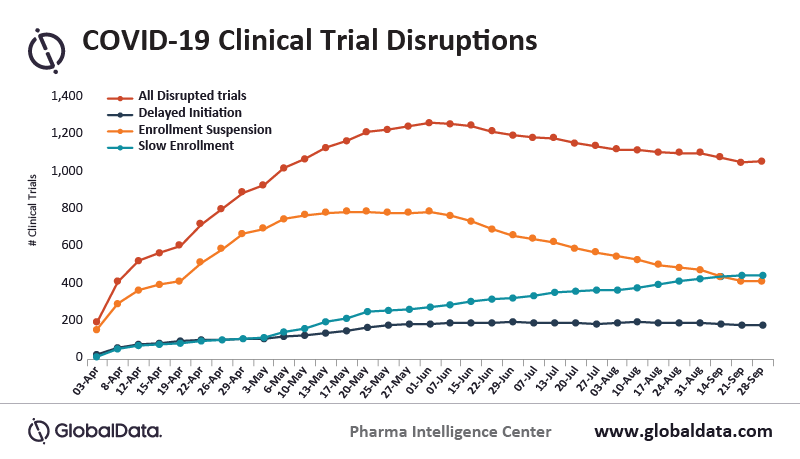
Trials impacted by enrollment suspension have been on a downward trajectory, while the number of clinical trials with delayed initiation have remained steady. This suggests that trials that had initiated enrollment before the pandemic with chosen sites and investigators but were then suspended due to COVID-19, are having more success picking up where they left off as long as enrollment was not impacted.
Wilson adds: “The total number of disrupted trials is falling and the number of clinical trials that have resumed continues to rise. This implies that sponsors and contract service providers have begun to adjust clinical trial design strategies and are adapting to the new post-COVID-19 environment.”























