Clinical trials are the foundation for getting needed drug and medical treatments to market. However, a new global survey found that disjointed technologies and undue complexity in sharing information and training is stalling outcomes.
“Sites are overwhelmed with too many solutions and ballooning login credentials,” said Jim Kremidas, executive director, ACRP. “Instead of engaging with patients, an inordinate amount of time is spent learning new systems, completing redundant required training and reentering the same data multiple times. Simultaneously, pharmaceutical companies and CROs are left with a fragmented ecosystem of software, hardware, vendors, and processes that ultimately distracts everyone involved from the goals of clinical research.”
The survey Impact Assessment of eClinical Technologies and Industry Initiatives on Sites commissioned by Oracle Health Sciences and conducted by the Society for Clinical Research Sites (SCRS) reveals a downward trend in site satisfaction with the current eClinical environment when participating in clinical trials. This is stifling their interest in new industry initiatives and new trial designs such as decentralized trials.
The survey indicates that while innovation in both clinical trial design and technology meant to improve the site and patient experience is available, investigative sites are concerned about the additional burden these technology changes bring in terms of training, complicated login processes, data duplication, etc. At the same time, sites are concerned about how to strengthen collaboration with sponsors and contract research organizations (CROs), while creating a competitive edge through improved clinical trial performance.
One third of respondents believe today’s eClinical solutions are not meeting their site’s operational needs. This represents a significant increase from 23 percent reported previously in research conducted by the Association of Clinical Research Professionals (ACRP)/CenterWatch in 2016.
The survey also revealed that there are many technology options and initiatives to address concerns raised by sites, yet 70 percent of respondents have no plan to use such technology due to high cost and complicated implementation processes.

When asked what technology features would help bridge the gap in meeting the site’s operational needs, respondents prioritized a single point of data entry (83 percent) and single sign-on (74 percent) as most important to streamline processes and eliminate redundant data entry.

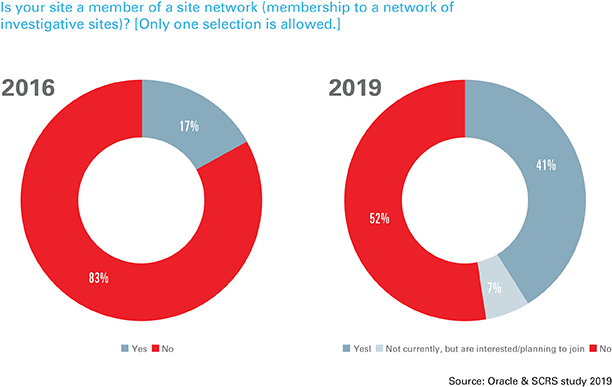
An increase in competition to participate in clinical research is encouraging sites to raise their profiles to increase their chances of being selected for studies. In fact, 48 percent of investigative sites (up 17 percent from the 2016 ACRP/CenterWatch survey) have or are planning to join site networks to gain a competitive edge.
In addition to improved trial access and site profile, respondents cited improved cycle time efficiencies (61 percent) and access to technology (54 percent) as the key benefits of joining site networks.
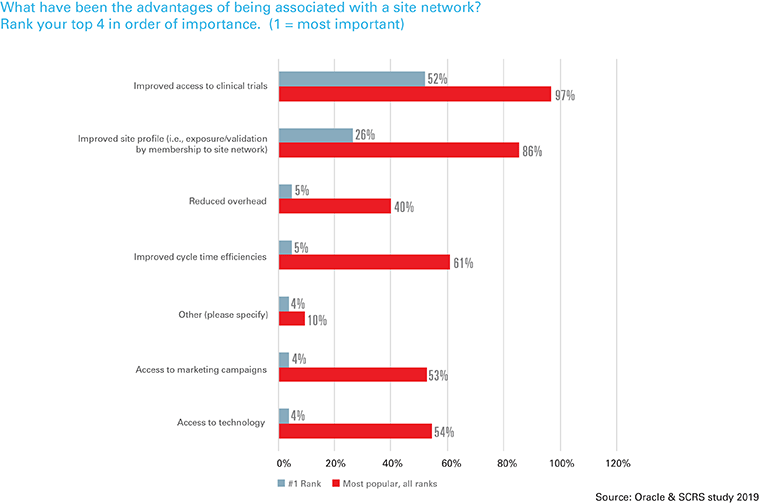
“There are significant barriers to entry for new sites, which is concerning given the ‘one and done’ syndrome plaguing the industry, which means a site completes a trial and does not pursue future studies,” said Casey Orvin, president, SCRS. “Site networks can help to reduce some of these burdens by standardizing procedures and enabling new sites to conduct studies, particularly in underrepresented urban areas and population clusters. Sponsors and technology vendors can help by simplifying the technology environment in which sites must work to participate in clinical research.”
The future of clinical trials
In addition to exploring how eClinical technologies affect the site experience, the survey sought to understand the impact of patient-centric study design, such as decentralized clinical trials.
The research probed into the site perspective on decentralized trials, defined as [direct-to-patient, or remote trials where patient data is partly collected offsite, for example in the patient’s home]. While respondents cited greater patient participation (82 percent) and patient access (71 percent) as positive anticipated benefits, they cited more systems to be trained in (69 percent), more usernames and passwords (62 percent), and fewer sites (47 percent) and staff (48 percent) as negative anticipated results. In addition, respondents indicated risk to overall study quality data as the number one concern with decentralized trials.
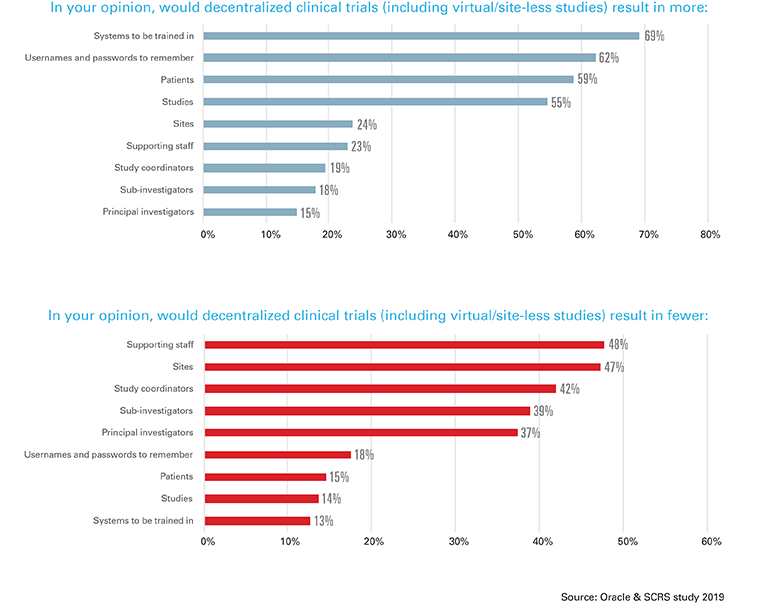
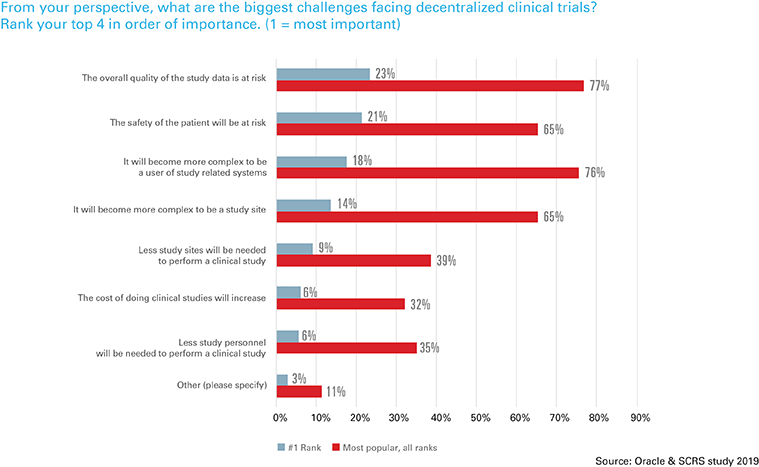
“We need to reimagine the way that software is consumed by all stakeholders across the clinical trial continuum. Software needs to be flexible and bring to bear the best functionality needed for clinical trials, regardless of modality,” said Steve Rosenberg, senior vice president and general manager of Oracle Health Sciences. “Sites are dynamic environments, and new technology and industry initiatives are an important complement to their relationship with sponsors and CROs. Understanding the sites’ perspective on these issues and addressing their frustrations is of paramount importance in guiding and sustaining clinical research globally.”
Download the full research report from here : Impact Assessment of eClinical Technologies and Industry Initiatives on Sites
























