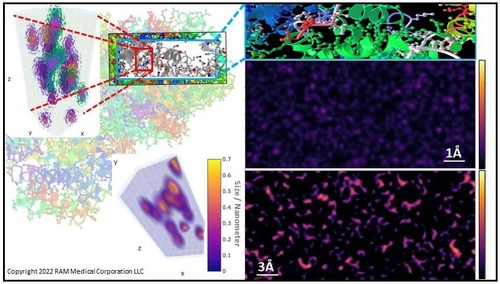
Revolutionary atomic-scale imaging has enabled non-destructive real-time observation of biomolecules within a protein in motion, the observation revealed how proteins use multi-frequency vibrations to change their shapes and functions, this new technology aims to revolutionize nothing less than drug development itself, dramatically reducing time and costs; understanding how life functions at the smallest scales can become our most powerful tool in the fight against all diseases—and the ability to identify underlying disease mechanisms.
DUBAI, UAE, Nov. 3, 2022 /PRNewswire/ — RAM Medical Corporation L.L.C. (RMC), based in UAE and Germany, has recently set up its headquarters in Dubai. For the past several years, RMC has been developing the next generation of biosensors for biomedical applications. As of October 2022, RMC innovation has created an IP portfolio of more than 60 sensor patents and operates its R&D facilities, cleanrooms, and research labs in Germany. The Company has developed a revolutionary 3D biomolecular mapping platform called ThEA™ (Terahertz Express Analyzer) for real-time 3D imaging of proteins in hopes of radically changing the drug development and personalized diagnostics industries.
A new perspective on “the small things”
RMC has been at the forefront of biotechnology breakthroughs in 3D structural imaging on the atomic scale. RMC’s biomolecular imaging platform ThEA™ was the first non-destructive technology to produce three-dimensional (3D) atomic-scale maps of entire portions of proteins in real-time. Recently, RMC announced a critical development that enables the 3D mapping of the entire inner and outer composition of proteins, including their higher-order structures.
Now, for the first time, our researchers can visualize the dynamic motions of individual protein segments, as well as the entire protein’s movements in biological samples. Considered one of the greatest challenges in structural biology or an impossible task by others, RMC is excited to announce the upcoming Q4 2022 ThEA™ product launch.
This new technology can reduce diagnostic testing times for disease detection to a matter of seconds! The ThEA™ technology has already been independently tested and clinically validated through patient trials in several countries (including Israel, Germany, and Saudi Arabia), and has received “CE” regulatory approval for clinical diagnostics of multiple viral pathogens throughout Europe.
The drug revolution starts here
Only 20% of new drugs successfully complete clinical trials, while timelines surpass 10 years, costing over $2 billion for a market-ready drug.
“In silico modeling,” such as those used to artificially model proteins and cells for drug development applications, have resulted in substantial numbers of false leads for drug developers, and are known to produce flawed 3D protein models and predictions when compared to biomolecular imaging techniques.
In contrast, ThEA’s 3D protein-mapping platform uses non-destructive sensors to map the entire protein’s outer and inner sub-structures, with a resolution of individual atoms. It will be the first time 3D structural transitions can be mapped and observed in proteins providing considerable insight for drug development and diagnostic applications.
The key to the ThEA™ technology is the application of patented Terahertz (THz) and Infrared (IR) sensors that detect, match, and combine a series of optical resonances and vibrational modes that are naturally occurring in proteins, and are translated into a 3D map of the protein.
“It was believed that having the ability to image proteins at a resolution of individual atoms was the holy grail in structural biology,” says Ayal Ram, the visionary CEO of RMC “however, observing a protein in action is the key to truly transforming the drug development, precision medicine, and personalized diagnostics industries.”
Small and intelligent
The ThEA™ platform uses AI-machine-learning models that combine both the physical and virtual to produce new functional drugs that will address unique disease models on an individual basis and enable tailoring drug designs to an individual’s ThEA-identified “underlying disease condition.”
The advancements in AI will fulfill a key role in discovering the best disease attack vectors for doctors, improving the decision-making process, and helping fill gaps in unknown disease models, and suggesting novel treatments.
Nothing less than the corona pandemic has given proof of ThEA’s disruptive platform with the capability to quickly develop and validate diagnostic tools for rapid disease detection, and diagnosis of individual disease mechanisms. “Using our ThEA™ platform based on a chemical-free and reagent-free direct detection method, we could differentiate infectious from non-infectious Covid-19 patients by analyzing the structures of three Covid-19 viral proteins and their collective 3D structures simultaneously—a capability not possible with any other technology.
Breaking down barriers to healthcare access
Recently, RMC developed two rapid diagnostic tests for Covid-19 and Influenza A/B with an all-in-one single test. The clinical results for both Covid-19 and Influenza were outstanding, achieving an overall accuracy equivalent to RT-PCR testing methods based on independent clinical studies and validations,” says Ayal Ram. “To date, RMC’s ThEA’s RapiDx™ Covid-19 Test continues to be the fastest diagnostic test for pathogens diagnostics worldwide, with a total measurement time of only 30 seconds.”
RAM was awarded the XPRIZE for its Covid-19 Test (a winner, and one of the four winning teams in the XPRIZE Rapid Covid-19 Testing Competition in 2021, “RAM ThEA™ platform announced a winner of XPRIZE Open Innovation Track“), also received CE regulatory approval, and is listed on the European Database on Medical Devices “EUDAMED,” (link: ThEA™ Device Analyzer and the link: ThEA™ Rapid Sensor), and is officially listed on the approved EU COVID-19 In Vitro Diagnostic Devices and Tests Database.
It’s crystal clear
Independent human clinical trials in Israel for Covid-19 results: Overall accuracy of 97.8% (Sensitivity 95.1%, Specificity 98.6%); and the independent human clinical trials in Israel for Influenza A/B results: Overall accuracy of 98.5% (Sensitivity 97.3%, Specificity 100%).
With the validation of its ThEA™ platform, RAM Medical Corporation is addressing a $60 bn laboratory equipment market, expected to continue having a double-digit growth over the next ten years.
About RMC: Ram Medical Corporation LLC, was founded by (a cancer survivor) Ayal Ram with the life goal of revolutionizing the biomedical diagnostic sector. RMC has a strong multi-disciplinary team who develop a wide array of biosensors for novel applications for over a decade. “We are constantly looking for ways to integrate our technologies to create new product platforms and disruptive applications.” says Ayal Ram, whose life and work motto is “All you can imagine is possible! It just has not been invented yet.“
Contact Details:
For Press & Media, email us @:
[email protected] or [email protected]
For Business Opportunities or Product Details:
[email protected] or [email protected]
For direct contact, call: +49-681-3096-4800
www.ramsolutions.com or www.ramglobal.com
SOURCE RAM Medical Corporation L.L.C.